|
|
|

Download a data sheet
|
Characteristics
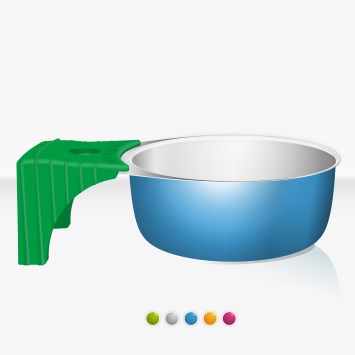
Each Maxicup® individual blister package contains:
- One sterile cooker
-
Maximum capacity of 5 ml. Allows to heat solutions of 3.5 ml easily
- Greater stability ensured by a plain surface
- Alloy of aluminium, which safety has been proven
-
Due to the material used and its relatively low thickness, the Maxicup becomes flimsy after the first use, to prevent from reusing it.
- The overmoulded plastic handle:
- reduces manipulations before use, and therefore the risk of handborne bacterial contamination, potential source of infections,
-
protects fingers from burns,
- ensures a greater stability.
- One sterile cotton filter:
- Due to its small size, this cotton filter retains less active drug than cigarette filters and makeshift filters alike.
-
Its high density also ensures an improved filtration efficacy
- Encourages single use of sterile filters
- One dry post-injection swab:
- To compress the vein after injection, for a better healing and to preserve the venous capital.
- Absorbs blood and reduces hand-borne viral transmission
Clinical history
The Maxicup has been developed in France since 2011 with the commitment of needle exchange programs. Its safety and efficacy have been validated through laboratory testing using crushed tablets and capsules.
Since September 2019, it is equipped with an overmoulded handle.
Maxicup® is a CE registered medical device.
|
|
|
|
|